Published online: 24 October 2016
J Gastrointest Surg (2017) 21:339-343
DOI 10.1007/s11605-016-3309-6
Is Revision Surgery Justified for Symptomatic Pancreaticoenteric Anastomotic Stenosis in Long-term Survivors Following Pancreaticoduodenectomy for Malignancy?
Prasad Wagle, Kamal Sunder Yadav, Priyanka Akhilesh Sali, Raman Garg & Paresh Varty
Abstract:
Background Pancreatico-enteric anastomotic (PEA) stenosis is one of the late complications following pancreaticoduodenectomy (PD) and reported for benign diseases. Literature for PEA stenosis following PD for malignancy is very limited due to low survival.
Material and Methods Patients undergoing surgery for symptomatic, recurrent, obstructive pancreatitis due to PEA stenosis following PD for malignancy were retrospectively identified from the authors’ prospective database between January 1997 and December 2014.
Results Six patients with median age 56.5 years underwent revision surgery for PEA stenosis during this time period. At primary PD, all were node negative with T1/T2 disease. The primary PEA were pancreatico-jejunostomy (PJ) (n = 5) and pancreaticogastrostomy (n = 1). Median time to develop symptoms was 62 months. At revision surgery, a Roux-en-Y longitudinal PJ (n = 5) and an end-to-side PJ (n = 1) were done. With a median follow-up of 36 months, pain relief was excellent (n = 5) to average (n = 1). Conclusion With improving long-term survival in patients undergoing PD for malignancy more such patients will be identified in future. Patients with symptomatic PEA stenosis following PD for malignancy can be managed surgically, with excellent outcomes in centers of expertise in pancreatic surgery.
Introduction:
With advances in surgical management and imaging techniques, mortality rate of pancreaticoduodenectomy (PD) has come down to less than 5 % in high-volume centers; however, morbidity rates remain significantly high.1 Immediate complications have been widely studied; however, late complications have not been well discussed.2 Stenosis of pancreatico-enteric anastomosis (PEA) following PD is one of the long-term complications and can present with symptomatic, recurrent, obstructive pancreatitis. Due to poor survival following PD for malignant lesions, the natural course of a PEA over time is not well documented. Also, the appropriate management of this condition is not yet outlined. Few studies have reported the role of revision surgery for PEA stenosis following PD in chronic pancreatitis; however, none have addressed its role in malignancy.2 A surgical correction of this long-term sequela is technically demanding and requires considerable surgical expertise due to altered anatomy. We highlight the natural course of PEA and share our experience of revision surgery for symptomatic anastomotic stenosis after PD for malignant lesions. To our knowledge, this is the first study reporting correction of PEA stenosis post PD for malignant etiology.
Material and Methods:
Over the 18-year study period (Jan 1997–Dec 2014), 308 PDs were performed for malignant disease at a high-volume, tertiary-care center. Technical information regarding primary surgery, post-operative period, and later presentation of PEA stenosis and its management were collected. Six patients (male/female; 4:2 with median age 56.5 years) eventually presented with a PEA stenosis, with recurrent abdominal pain and documented pancreatitis. All PDs were done by two surgeons (first and last authors) skilled in hepaticopancreatico-biliary surgery, except for two patients who were operated by different surgeons at different institutes. Indications for index surgery were ampullary carcinoma (n = 3), distal CBD cholangiocarcinoma (n = 1), neuroendocrine tumor of pancreatic head (n = 1), and chronic pancreatitis with a small head adenocarcinoma (n = 1). All resected specimens were node negative with T1/T2 disease. During the primary PD, out of six patients, five had pancreaticojejunostomy (PJ) and one had a pancreatico-gastrostomy (PG). There was no documented pancreatic leak following primary surgery in these six patients. Asymptomatic patients with ductal dilation or glandular atrophy on follow-up imaging, or with newly onset diabetes, were not included in our study. Symptomatic PEA stenosis was found in 1.3 % (4/308), excluding two patients who were originally operated by other surgeons.
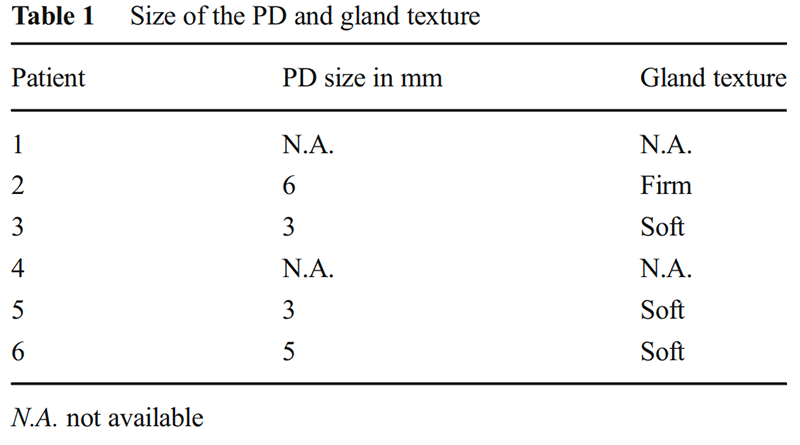
During primary surgery, PEA was performed either by the dunking technique or the duct-to-mucosa done in two layers. In the dunking technique, an outer 3-0/4-0 prolene material and an inner 5-0 polydioxanone material was used. In the duct-to-mucosa, an interrupted 3-0/4-0 prolene sutures were used for the outer layer, approximating jejunal serosa with pancreatic capsule and 5-0 or 6-0 polydioxanone interrupted sutures were taken radially for duct-to-mucosa anastomosis. Surgical loupes with a ×3.5 magnification were used for reconstruction from 2008. The duct size and gland texture in each case have been summarized in Table 1 (except case 1 and 4, where the primary surgery was performed at another institute). The size of the PD was a visual observation, and it was not measured with any scale. Prophylactic octreotide in the perioperative period was given for 5 days in all patients.
For these symptomatic patients (n = 6), investigations included routine blood work-up, serum amylase, serum lipase, CA 19-9, and computed tomography (CT) and/or magnetic resonance cholangio-pancreatography (MRCP) was done to visualize the PES, as also to rule out recurrence of disease. On imaging, PEA stenosis was identified as ductal narrowing and/or a fixed filling defect at the index PEA with upstream ductal dilatation, with or without side branch enlargement. All six patients were taken up for revision surgery as they failed conservative trial with enzyme supplementation and analgesics. Patients were closely observed during hospital stay and kept under close follow-up in OPD thereafter.
Results:
Out of the 310 (308 + 2) patients that underwent PD, six developed PEA stenosis (1.93 %). Of these, only one had undergone PG. The median time of presentation was 62 months (range 48–120 months). Endoscopic intervention was attempted in two of them but failed. This was due to the altered anatomy with difficult access to the anastomotic region. Five patients underwent Roux-en-Y lateral pancreatico-jejunostomy and one underwent end-to-side PJ. No anastomotic stents were used in any of the patients. Average blood loss was 175 ml with a range of (100 to 550 ml). Octreotide was used in all the patients. Postoperative pain was well managed with NSAIDS and narcotics. There was no mortality; however, morbidity was seen in 3/6 (50 %) patients. Of the three patients who had post-operative morbidity, one had transient ascites due to hepatitis C-related child A cirrhosis, one had delayed gastric emptying, and another developed grade A pancreatic fistula (Table 2). All were successfully managed conservatively. Oral feeds were started on the third post-operative day. All except one patient were discharged within 10 days. The patient with delayed gastric emptying was discharged on day 18.
Follow-up: On a median follow-up of 36 months (range 16–84 months), the pain relief was excellent in five patients and average in one patient. None of the patients had an endocrine or an exocrine insufficiency.
Discussion:
Due to improvised surgical techniques and sophisticated intensive care, mortality associated with PD has markedly reduced. However, morbidity due to the complications continues to be encountered significantly. PD is also done for premalignant and low-grade malignancies as well as for intraductal pancreatic mucinous neoplasms, gastrointestinal stromal tumors, neuroendocrine tumors, adenomas, and benign conditions. Pancreatic fistula and delayed gastric emptying are the commonly encountered early post-operative complications. PEA stenosis is a late complication, and data for which was scarce, possibly due to poor longevity associated with patients undergoing the procedure for malignancy. However, with PD being increasingly performed for even benign or low-grade tumors, a larger number of patients with this long-term complication may be studied.

Morgan et al. reported a PEA stenosis rate of 11 % in 237 patients who underwent pancreatic head resection for benign conditions.2 Demirjian et al. reported a symptomatic anastomotic stricture rate of 2 % over 357 patients who underwent PD for both benign and malignant conditions.3 ReidLombardo et al. had a PEA stenosis rate of 3.3 % in patients who underwent surgery for benign diseases.4 Few single case reports have also been reported.5– 7
It has been found that post-operative pancreatic fistula may probably lead to PEA stenosis due to extensive fibrotic changes and the hostile peri-anastomotic environment created due to the pancreatic enzyme exposure.3 Also, if chronic pancreatitis was present complicating the condition for which the primary procedure was done, chances of PEA stenosis were higher.2 The consistency of the gland during the primary surgery was also thought to be associated but no conclusive relationship was found.3 It was also contemplated that a duct of smaller diameter could lead to the anastomotic stricture.3 However, post-operative pancreatic fistulas have the highest chances of causing the anastomotic stricture. PEA stenosis may occur several years after the primary procedure and patients may also be asymptomatic. However, stenosis may be seen as early as 1 to 2 weeks post-operatively, and this has been attributed to the intense inflammation and fibrosis surrounding the anastomotic site.7,8 The cases reported thus far have been briefly summarized in Table 3.

-as-also-a-double-duct-hepatico-jejunostomy-(B).png)
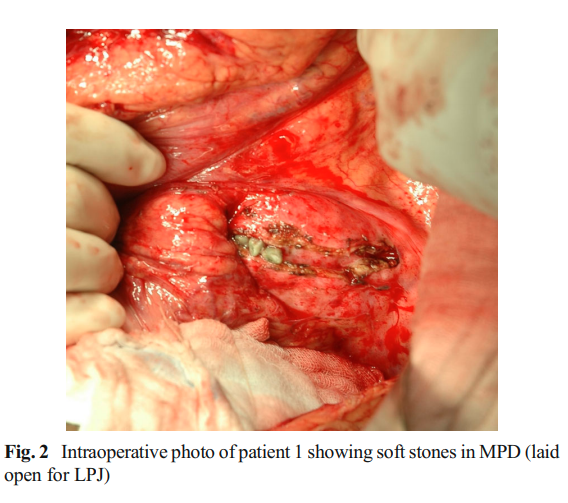
None of our patients in this series had documented pancreatic leaks after the primary surgery unlike the incidence reported by Morgan et al.2 and Demirjian et al.3 We would speculate that there could have been some delayed marginal vascular compromise at the anastomotic site to not cause a pancreatic leak but a subsequent stricture in the long run although there also exists a possibility of a subclinical pancreatic leak leading to a subsequent PES. Another factor could also be inspissated jejunal/gastric secretions in the duct leading to duct blockage. Three of our patients had inspissated soft stones [Fig. 1 and 2] at the anastomotic site though with a narrowed anastomosis. Patient 1 had hepatitis C-related child A cirrhosis at the time of the revision surgery. The transient ascites that he had did not preclude an early recovery. Patient 5 had a bifid pancreatic duct (missed during the index surgery) [Fig. 3]. The pancreatic edge here was refashioned to expose both the ducts [Fig. 4]. The adjacent duct walls were sutured to create a single orifice, and a duct-to-mucosa anastomosis was fashioned.
Patients with PEA stenosis generally present with chronic abdominal pain. Since these patients are on supplementary pancreatic enzymes, steatorrhea is not commonly seen.2 Other possible causes of abdominal pain need to be excluded, and those with elevated pancreatic enzymes must be further investigated. Magnetic resonance cholangiopancreaticography (MRCP) should be performed in suspected cases of PEA stenosis causing pancreatitis. This was done in all the patients in our study. Some have also reported the use of secretinstimulated MRCP in demonstrating the anastomotic stenosis, however, without any added advantage over a conventional MRCP.9,10 A diagnosis of PEA stenosis was made if there was significant ductal narrowing and/or a filling defect seen at the site of pancreatico-enteric anastomosis with upstream ductal dilatation and side branch enlargement. Conservative mode of therapy may be tried, but generally fails in a PEA stenosis causing pancreatitis. Endoscopic balloon dilatations with stent placements have been tried.4 However, this may not be always possible. Altered anatomy due to the previous surgery may hinder the visualization and further therapeutic intervention. Endoscopic intervention was unsuccessfully attempted in two of our patients.
-in-the-bifid-pancreatic-ducts-and-artery-forceps-in-the-jejunal-orifice.png)
Surgical repair of PEA stenosis is technically challenging due to dense fibrosis and adhesions. Intraoperative ERCP and/ or IOUS may also be used. If the stenosis is identified, a portion of the remnant pancreas is also resected to achieve a dilated pancreatic duct for a new PEA. Sometimes, due to tight adhesions, visualization of the stricture site is not possible, and in these cases, a trans-jejunal stricturoplasty may be done.3 A conventional lateral pancreatico-jejunostomy may be done if the pancreatic duct is widely dilated due to obstruction.
In our study, all the six patients who developed a pancreatico-enteric stenosis underwent a surgical repair with an acceptable outcome. Complications of a pancreatic fistula (grade A), DGE and transient ascites were seen in three patients that were managed conservatively.
Conclusion:
PEA stenosis is a rare cause of post-PD pancreatitis. MRCP is helpful in identifying the stenosis. Surgical intervention is helpful in alleviating the debilitating symptoms of pancreatitis. However, this operative endeavor requires significant expertise and is technically demanding. Our experience suggests a good and satisfying outcome for most our patients with the condition and can be undertaken in specialist centers.
- Jakhmola CK, Kumar A. Whipple’s pancreaticoduodenectomy: Outcomes at a tertiary care hospital. Medical journal, Armed Forces India. 2014; 70(4):321-6.
- Morgan K, Fontenot B, Harvey N, Adams D. Revision of anasto- motic stenosis after pancreatic head resection for chronic pancrea- titis: is it futile? HPB. 2010; 12(3):211-216.
- Demirjian A, Kent T, Callery M, Vollmer C. The inconsistent nature of symptomatic pancreatico-jejunostomy anastomotic strictures. HPB. 2010; 12(7):482-487.
- Reid-Lombardo KM, Ramos-De la Medina A, Thomsen K, Harmsen WS, Farnell MB. Long-term anastomotic complications after pancreaticoduodenectomy for benign diseases. J Gastrointest Surg. 2007; 11(12):1704–1711.
- Kuroki T, Tajima Y, Tsutsumi R, Adachi T, Kitasato A, Hamasaki K et al. S ur gical man a gemen t for s teno s i s o f t he pancreaticojejunostomy. Int Surg.2008; 93(3):155–157.
- Menon K, Sanaka M. Successful Single-Balloon Enteroscopic Dilation of Late Anastomotic Pancreaticojejunostomy Stricture Following Whipple Procedure. Pancreas. 2010; 39(1):115-116.
- Seelig M, Janot M, Chromik A, Herzog T, Belyaev O, Weyhe D et al. Redo-Surgery following Curative Resection of Pancreatic Carcinoma: The Difference between True and Suspected Recurrence. Digestive Surgery. 2009; 26(3):222-228.
- Kurosaki I, Hatakeyama K, Nihei K. Pancreaticogastrostomy: un- reliable long-term pancreatic duct patency. Hepatogastroenterology 2003; 50(50):545-549.
- Sho M, Nakajima Y, Kanehiro H, Hisanaga M, Nishio K, Nagao M et al. A new evaluation of pancreatic function after pancreaticoduodenectomy using secretin-stimulated MRCP. Am J Surg. 1998; 178(3):279–282.
- Nordback I, Parviainen M, Piironen A, Raty S, Sand J. Obstructed pancreaticojejunostomy partially explains exocrine insufficiency after pancreatic head resection. Scand J Gastroenterol. 2007; 42(2):263–270.