Published online: 12 August 2019
Desai GS, et al. BMJ Case Rep 2019;12:e228156. doi:10.1136/bcr-2018-228156 3
Late postcholecystectomy Mirizzi syndrome due to a sessile gall bladder remnant calculus managed by laparoscopic completion cholecystectomy: a feasible surgical option
Gunjan S Desai, Prasad Pande, Rajvilas Narkhede, Prasad Wagle
Summary:
Postcholecystectomy Mirizzi syndrome (PCMS) is an uncommon entity that can occur due to cystic duct stump calculus, gall bladder remnant calculus or migrated surgical clip. It can be classified into early PCMS or late PCMS. It is often misdiagnosed and the management depends on the site of impaction of stone or clip. Endoscopy can be performed for cystic duct stump calculus. However, surgery is the treatment for remnant gall bladder calculus. Role of laparoscopic management is controversial. We present here a case of a 48-year-old woman with late PCMS due to an impacted calculus in a sessile gall bladder remnant following a subtotal cholecystectomy, managed with laparoscopic completion cholecystectomy, review the literature, provide tips for safe laparoscopy for PCMS and summarise our algorithmic approach to the management of the postcholecystectomy syndrome.
Background:
Postcholecystectomy syndrome (PCS) commonly occurs due to extrabiliary causes such as peptic ulcer disease, reflux oesophagitis, irritable bowel syndrome, hepatitis and chronic pancreatitis. Biliary causes such as retained or recurrent biliary calculi with/out cholecystitis, biliary stricture, biliary dyskinesia and biliary leaks cause early or late PCS less frequently as compared with extrabiliary causes. Among all biliary causes of late PCS, postcholecystectomy Mirizzi syndrome (PCMS) is extremely rare.1–3 It is often misdiagnosed and difficult to manage. Hence, all attempts should be made in the index surgery to prevent PCMS.
PCMS can occur due to cystic duct stump calculus or gall bladder remnant calculus. The role of laparoscopy in its management is controversial.1–4 We present a case wherein, laparoscopic completion cholecystectomy (LCC) was performed for PCMS due to gall bladder remnant calculus, review the literature and summarise our algorithmic approach to the management of PCS.
Case presentation:
A 48-year-old woman presented with recurrent acute right upper abdominal pain, nausea and vomiting episodes since a month. She had undergone emergency open subtotal cholecystectomy (SC) for acute calculous cholecystitis 18 years ago, mitral valve replacement 7 months ago and was on oral anticoagulation.
Investigations:
On investigation, alkaline phosphatase was elevated. Other liver function tests (LFT) were within normal limits. Transabdominal ultrasound revealed a walled-off collection in gall bladder fossa with a non-obstructing common bile duct (CBD) calculus. MR cholangiopancreatography (MRCP) revealed a gall bladder remnant with a calculus within, compressing the common hepatic duct (CHD) suggestive of the type 1 Mirizzi syndrome (MS) (figure 1).
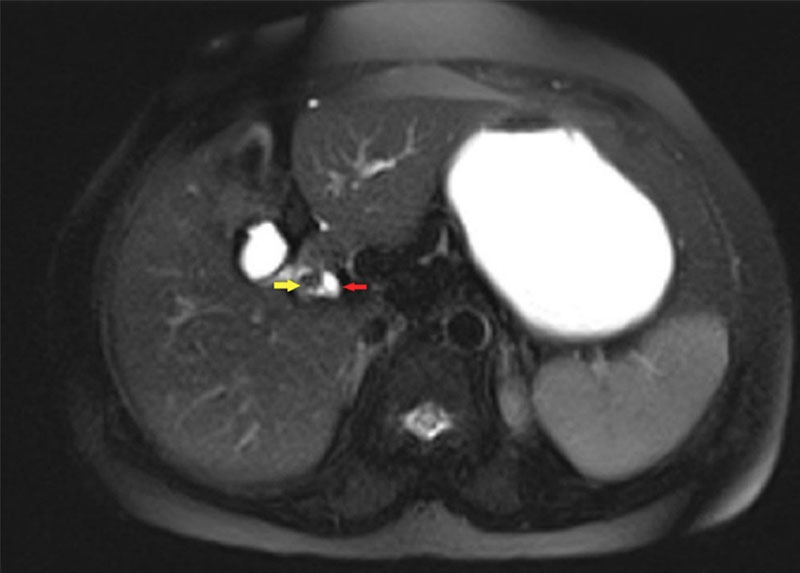
Figure 1 Axial MRI T2 half Fourier single-shot turbo spin echo image showing the stone in remnant gall bladder (yellow arrow) compressing the common hepatic duct (red arrow).
Treatment:
LCC was planned with consideration of a high probability of conversion to open surgery. During surgery, utmost care was taken to identify Rouviere’s sulcus and keep the dissection above it, following a right-to-left dissection. The globular inflamed sessile gall bladder remnant, containing a calculus impacted at Hartmann’s pouch, was found densely adherent to the wall of CHD. CBD was normal. Calot’s triangle was defined, and a combination of retrograde and antegrade cholecystectomy was performed to reach the remnant gall bladder–CBD junction (figures 2, 3). The remnant was separated from the CHD, taking care not to injure the CHD. No cholecystocholedochal fistula was identified. The remnant gall bladder and cystic artery were clipped and divided after attaining the critical view of safety, using a combination of sutures and clips. Histopathology revealed chronic cholecystitis.
Outcome and follow-up:
The patient recovered uneventfully and is doing well at 5-month follow-up.
Discussion:
PCS comprises a heterogeneous group of symptoms and signs in a patient after cholecystectomy.1 Its overall incidence is 10%–40% with female preponderance (43% in women versus 28% in men).2 It has been reported from 2 days to 32 years after cholecystectomy.3 The myriad symptoms and signs often hint toward the probable cause of PCS. The diagnostic workup depends on these symptom clusters as can be seen in figure 4 which summarises the clinical presentation, causes and our approach to the diagnosis of PCS.

Figure 3 Intraoperative image showing the common bile duct–gall bladder junction (blue arrow) and the right hepatic duct (yellow arrow). The plane of posterior dissection is above the Rouviere’s sulcus.
The major risk factor for biliary PCS is SC, which occurs in up to 13.3% of cases of laparoscopic cholecystectomy (LC). SC is performed intentionally in cases with poor visualisation, severe inflammation or adhesions in Calot’s triangle, cholelithiasis in portal hypertension or cirrhosis, and MS, to avoid bile duct injury and excessive bleeding. It may also be performed unintentionally as a result of congenital duplication or an hourglass configuration due to adenomyomatosis, especially by inexperienced surgeons.4 5
As seen in figure 3, early PCS occurs within 2 years of cholecystectomy. 1 Among cases of late PCS, remnant stones in cystic duct stump account for 17%–25% cases.6 A cystic duct stump >1cm in length after cholecystectomy causing PCS is known as cystic duct stump syndrome.7 Palanivelu et al8 reported an incidence of remnant cystic duct calculus of 4.19% in LC as compared with 0.02% in open cholecystectomy.9
PCMS can occur due to stone within gall bladder remnant or cystic duct stump or due to surgical clip.9 10 It can be associated with an atrophic gall bladder remnant with thick/thin walls, impacted gallstones at the infundibulum or Hartmann’s pouch, an obstructed long or short cystic duct with or without a low insertion, partial/complete obstruction by external compression of CHD by a gallstone or surgical clip, and occasional presence of an anomalous communication of gall bladder remnant or cystic duct stump with CBD or small intestine.10 11 It can present with any of the biliary symptoms shown in figure 3. Across studies, 75%–80% patients have deranged LFT, most commonly, an elevated gamma-glutamyl transferase.
In one study involving 198 patients, the sensitivity of ultrasound for MS was as high as 77.8%.12 Though endoscopic
retrograde cholangiopancreatography (ERCP) is the gold standard for MS, MRCP is a highly sensitive non-invasive diagnostic investigation with sensitivity approaching 100% and is preferred over ERCP.3 Heavily T2-weighted axial MR images can easily demonstrate the closely lying cystic duct and CHD which may sometimes be missed on MRCP.6 Endoscopic ultrasound can also be performed prior to ERCP.12 Percutaneous transhepatic cholangiography and intraductal ultrasonography for identification of fistula are also options.13–15
Management of PCMS is complicated by the presence of dense adhesions in Calot’s triangle and subhepatic region due to longstanding inflammation, which increases the risk of bile duct injury or significant bleeding. Complications of MS include biliary cutaneous fistula, secondary biliary cirrhosis and lateonset biliary strictures, which also make the surgical management difficult.12 The management depends on whether the stone is in cystic duct stump or gall bladder remnant.
For cystic duct stump calculus leading to PCMS, endoscopic management is feasible with options including sphincterotomy and balloon or basket use/holmiumlaser lithotripsy, electrohydraulic lithotripsy, mechanical extracorporeal shockwave lithotripsy for stone fragmentation or chemical dissolution followed by retrieval of stone fragments endoscopically.3 6 In remnant gall bladder after SC with calculus in the cystic duct, endoscopic manipulation of stone back into the gall bladder followed by LCC is also an option.6 However, endoscopic retrieval of retained gall bladder remnant calculus should not be attempted.3
PCMS due to gall bladder remnant calculus can be managed by a preoperative ERCP with sphincterotomy and stent followed by LCC or open CC. Single-stage LC is also an option, to be attempted by experienced laparoscopic surgeons at advanced laparoscopy centres.3 In a study, preoperative drainage with an endoscopic stent, or a nasobiliary drain placement by ERCP, was used to facilitate surgery.12 The advantage of preoperative ERCP is that it allows for the acute inflammatory stage to resolve, thereby reducing the difficulty of the surgery, and the resulting morbidity. The disadvantage is the addition of an invasive procedure, with its inherent risks and complications.
CC is feasible in cases of type 1 MS due to any cause and is the treatment of choice to avoid the above-mentioned complications of MS unless the patient is unfit for surgery.3 12 In that scenario, endoscopic management is preferred. After SC, intense inflammation and dense adhesions make identification of cystic duct and Calot’s triangle difficult. Hence, LCC is not routinely attempted.4 However, with growing expertise, laparoscopic management is considered safe and feasible for type 1 MS, even in postcholecystectomy patients with MS due to remnant gall bladder calculus. In a recent study by Sanjay Kumar Saroj et al, the re-exploration rates in laparoscopic and open CC for type 1MS were found similar. 1
During LCC for PCMS, care should be taken to remain above the Rouviere’s sulcus, achieve the critical view of safety, dissect from right to left to avoid the bile duct and minimise the use of energy devices in Calot’s triangle. A low threshold should be kept for conversion to open surgery. In contrast, prevention of PCS due to gall bladder remnant or cystic duct stump stone is possible by meticulous dissection of the cystic duct up to its junction with bile duct followed by the milking of duct before clipping. Liberal use of intraoperative cholangiogram delineates the anatomy well and avoids bile duct injuries, in addition to identifying any residual CBD stones missed on MRCP. Achieving the critical view of safety is of paramount importance to avoid bile duct injury and related causes of PCS. Also, the cystic duct stump should not be kept >5mm long when a cystic duct stump is present.3 4
Learning points:
- Postcholecystectomy Mirizzi syndrome (PCMS), although rare, should be one of the differential diagnoses in patients presenting with postcholecystectomy syndrome.
- Axial MR images should be studied since coronal MR cholangiopancreatography images may miss subtle compression of common hepatic duct.
- Laparoscopic completion cholecystectomy is a safe and feasible option for type 1 PCMS when carried out by experienced surgeons.
- The threshold for conversion to open surgery should be low in such cases.
Contributors GSD, RN and PP: worked for the literature review and the manuscript
preparation under the creative inputs, critical evaluation and guidance of PW. All
authors have worked on the article generation and editing.
Funding The authors have not declared a specific grant for this research from any
funding agency in the public, commercial or not-for-profit sectors.
Competing interests Not declared.
Patient consent for publication Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
References:
- Saroj SK, Kumar S, Afaque Y, et al. The Laparoscopic Re-Exploration in the Management of the Gallbladder Remnant and the Cystic Duct Stump Calculi. J Clin Diagn Res 2016;10:PC06–PC08.
- Nagorni EA, Kouklakis G, Tsaroucha A, et al. Post-laparoscopic cholecystectomy Mirizzi syndrome induced by polymeric surgical clips: a case report and review of the literature. J Med Case Rep 2016;10:1–7.
- Amin A, Zhurov Y, Ibrahim G, et al. Combined Endoscopic and Laparoscopic Management of Postcholecystectomy Mirizzi Syndrome from a Remnant Cystic Duct Stone: Case Report and Review of the Literature. Case Rep Surg 2016;2016:1–9.
- El Nakeeb A, Ezzat H, Askar W, et al. Management of residual gallbladder and cystic duct stump stone after cholecystectomy: a retrospective study. The Egyptian J Surg 2016;35:391.
- Chowbey P, Sharma A, Goswami A, et al. Residual gallbladder stones after cholecystectomy: A literature review. J Minim Access Surg 2015;11:223.
- Wani NA, Khan NA, Shah AI, et al. Post-cholecystectomy Mirizzi’s syndrome: magnetic resonance cholangiopancreatography demonstration. Saudi J Gastroenterol 2010;16:295–8
- Shirah BH, Shirah HA, Zafar SH, et al. Clinical patterns of postcholecystectomy syndrome. Ann Hepatobiliary Pancreat Surg 2018;22:52–7.
- Palanivelu C, Rangarajan M, Jategaonkar PA, et al. Laparoscopic management of remnant cystic duct calculi: a retrospective study. Ann R Coll Surg Engl 2009;91:25–9.
- Kodali VP, Petersen BT. Endoscopic therapy of postcholecystectomy Mirizzi syndrome. Gastrointest Endosc 1996;44:86–90.
- Beltrán MA. Mirizzi syndrome: history, current knowledge and proposal of a simplified classification. World J Gastroenterol 2012;18:4639–50.
- Beltran MA, Csendes A, Cruces KS. The relationship of Mirizzi syndrome and cholecystoenteric fistula: validation of a modified classification. World J Surg 2008;32:2237–43.
- Chen H, Siwo EA, Khu M, et al. Current trends in the management of Mirizzi Syndrome: A review of literature. Medicine 2018;97:e9691.
- Wehrmann T, Riphaus A, Martchenko K, et al. Intraductal ultrasonography in the diagnosis of Mirizzi syndrome. Endoscopy 2006;38:717–22.
- Moon JH, Cho YD, Cheon YK, et al. Wire-guided intraductal US in the assessment of bile duct strictures with Mirizzi syndrome-like features at ERCP. Gastrointest Endosc 2002;56:873–9.
- Kim DC, Moon JH, Choi HJ, et al. Successful endoscopic treatment for Mirizzi syndrome type II under direct peroral cholangioscopy using an ultraslim upper endoscope. Endoscopy 2014;46(Suppl 1):E103–E104.

