Date: 25 February 2019
Doi:10.1136/bcr-2018-228510
Chavan N, et al. BMJ Case Rep 2019;12:e228510.
Intrapancreatic accessory spleen: an enigmatic entity
Namita Chavan, Gunjan Shailesh Desai, Chandralekha Tampi, Prasad Wagle
1. Department of Gastrointestinal Surgery, Lilavati Hospital and Research Centre, Mumbai, India
2. Department of Pathology, Lilavati Hospital and Research Centre, Mumbai, India
Summary:
Solitary hypervascular lesion in the distal body/tail of pancreas in a patient with non-specific abdominal symptoms is a diagnostic challenge. Neuroendocrine neoplasm (NEN) and metastasis from renal cell carcinoma are the most common differentials and intrapancreatic accessory spleen (IPAS) is the rarest of its differential diagnosis. We present, here, a case of a 56-year-old man with a space-occupying lesion in body/ tail of pancreas that was preoperatively diagnosed as a NEN based on elevated chromogranin levels and hyperenhancing lesion on contrast-enhanced CT scan. He underwent a spleen-preserving distal pancreatectomy. The final histopathology revealed an IPAS.
Background:
Intrapancreatic accessory spleen (IPAS) is a rare differential diagnosis of a solitary hypervascular lesion in body/tail of pancreas.1 2 A literature review reveals only around 105 cases worldwide as per a search conducted on MEDLINE.3 The entity is very important to suspect preoperatively because we can avoid surgery, if it is detected with certainty. We present, here, a case of IPAS and an approach to diagnosis and management of these lesions.
Case presentation:
A 56-year-old man, with no comorbidities, presented with multiple episodes of non-colicky, non-radiating, dull epigastric pain since 2 months. He had 2–3 episodes of vomiting and nausea in the same duration. His appetite was preserved and he had no other abdominal conditions. He was a smoker and consumed alcohol occasionally since last 30 years.
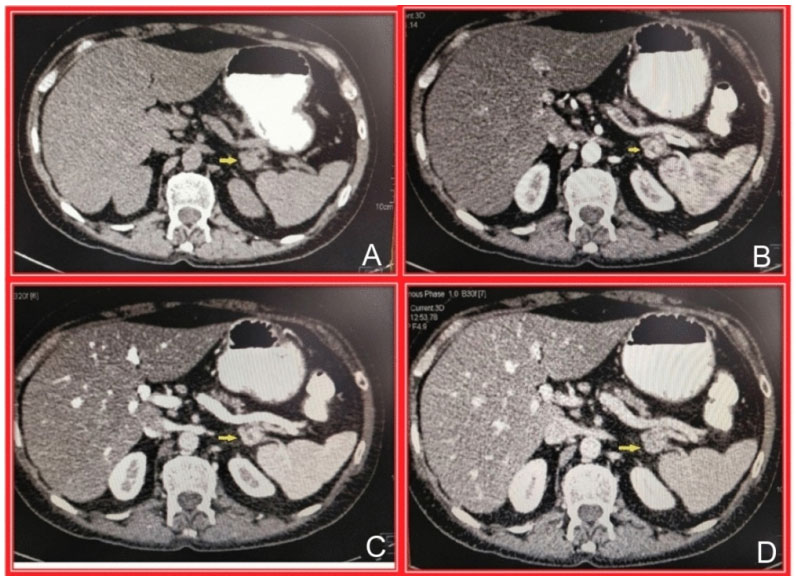
Figure 1 (A) Non-contrast CT abdomen, axial image, showing the lesion in distal body of pancreas, no calcification; (B) Contrast-enhanced CT (CECT) abdomen, arterial phase, axial image shows the hyperenhancing lesion in distal body of pancreas; (C) CECT abdomen, portal phase, axial image shows the washing out of the lesion in distal body of pancreas and (D) CECT abdomen, venous phase, axial image shows washout of the contrast from the lesion in distal body of pancreas.
Investigations:
Complete blood counts, liver function tests, amylase and lipase were normal. He had an elevated chromogranin A level of 168ng/L (normal: <39ng/L, not on proton pump inhibitors).
Abdominal contrast-enhanced CT (CECT) scan showed a 2×1×1cm hyperenhancing lesion in distal body and tail of pancreas suspicious of neuroendocrine neoplasm (NEN) (figure 1).
Differential diagnosis:
The classical differential diagnosis includes a NEN, hypervascular metastasis to pancreas and IPAS. However, the presentation was very highly suggestive of NEN and our imaging diagnosis was NEN.
Treatment:
The patient refused further evaluation by endoscopic ultrasound-guided biopsy or nuclear imaging to confirm NEN due to cost constraints and a spleen-preserving distal pancreatectomy was planned.
Intraoperatively, there was firm, atrophied pancreas with a single, approximately 2.3×1.5×1cm, tumour in the pancreatic tail. Intraoperative ultrasound did not reveal any new lesions. A spleen-preserving distal pancreatectomy was performed. On gross histopathological examination, distal pancreas showed 1.5×1×1cm brownish encapsulated nodule with grey–white lobulated cut surface placed eccentrically within the body/tail of the pancreas. On microscopic examination, the nodule had few fibrocollagenous spurs. It was cellular with red pulp containing cords of Billroth, littoral cells, phagocytes, few plasma cells with small T lymphocytes and white pulp showing lymphoid nodules and follicles of B lymphocytes with eccentrically placed arterioles (figure 2). Immunohistochemistry (IHC) was negative for chromogranin and synaptophysin while positive for cluster of differentiation (CD) 3, CD20, CD31, CD68 and CD138. CD8 brilliantly lit up the littoral cells (figure 3). The morphology, supported by the IHC, confirmed the mass to be an IPAS. Rest of the pancreas showed early features of chronic pancreatitis.

Figure 2 H&E stain (×40 magnification) shows the red and white pulp, and the adjacent pancreatic tissue.
Outcome and follow-up:
Postoperative recovery was uneventful and the patient is doing well at 1.5-year clinical follow-up.
Discussion:
Accessory spleen is a congenital anomaly occurring due to non-union of migrated mesenchymal cells in dorsal mesogastrium during the fifth week of intrauterine life.1 This congenital anomaly leads to the appearance of normal splenic tissue at ectopic sites along the splenic vessels, wall of jejunum, in mesentery or even in pelvis. In an autopsy study conducted on 311 patients, the most common site of accessory spleen was splenic hilum (80%), followed by proximity to pancreatic tail (17%).2 To date, only 105 cases have been reported worldwide as per a search conducted on MEDLINE, of which 73% cases were detected incidentally.3
Pathologically, IPAS is 1–3cm round brownish nodule usually seen in the tail of the pancreas. On microscopic examination, it is thickly encapsulated cellular nodule showing characteristic red and white pulp. White pulp shows lymphoid nodules with follicles of B lymphocytes and cells of reticuloendothelial system with eccentrically placed arterioles. Red pulp is made up of numerous vascular sinuses, cords of Billroth, phagocytes, plasma cells and T lymphocytes.2 4
Multimodality imaging is essential for diagnoses of IPAS. On ultrasonography (USG), accessory spleen has a surrounding high-amplitude interface, which represents fibrotic capsule of the spleen.5 It is mildly echogenic, homogeneous with posterior enhancement. On colour Doppler, the characteristic finding of vascular hilum entering the lesion is seen, which is the diagnostic feature of accessory spleen with a sensitivity of 90%.5 To characterise various phases, microbubbles containing contrast agents, such as sulfur hexafluoride are used during contrast-enhanced USG (CEUSG). In delayed hepatosplenic phase, these microbubbles get trapped almost exclusively by splenic and hepatic parenchyma. Thus, CEUSG helps to identify accessory spleen.6 On CECT, it has density and intensity similar to the spleen in arterial portal venous/pancreatic parenchymal and venous/delayed phases. The lesion remains static on follow-up imaging.7 On MRI T1-weighted images, the lesion is hypointense, whereas it is hyperintense on T2-weighted fat-saturated images. The key point in diagnosis is that it's signal intensities are identical to those of spleen on multiple MR pulse sequences. In superparamagnetic iron oxide-enhanced MRI scan, the contrast is targeted to reticuloendothelial cells, which shows a signal drop in IPAS similar to that of spleen.5 7

Figure 3 Immunohistochemistry with cluster of differentiation 8 highlights the littoral cells, confirming a spleniculi.
When a patient presents with an asymptomatic or vaguely symptomatic solitary pancreatic lesion, various differential diagnoses are solid pseudopapillary tumour, islet cell tumour, pancreatic adenocarcinoma, endocrine tumour and metastasis. Among these, NEN and metastasis are more commonly hypervascular. Pancreatic tail lesion with an appropriate clinical history of primary malignancy, easily establishes the diagnosis of metastasis. Also, the majority of metastatic lesions are multiple, well-defined heterogeneous mass./p>
Almost 87% of patients with IPAS were suspected as NEN in a study.3 NEN on CECT also appears as a hyperenhancing lesion with/out lymphadenopathy. Non-functioning NENs are more common than functioning NENs and these can be easily confused with intrapancreatic spleen due to lack of symptoms.7–9 In these cases, chromogranin levels and nuclear medicine can be used as modalities to rule out NEN and establish the diagnosis of IPAS.
Chromogranin A is the most commonly used biomarker for NENs. Radioimmunoassay, enzyme-linked immunosorbent assay, western blot, immunofluorescent microscopy and immunoradiometric assay are the techniques used to assess serum chromogranin A levels. However, it is not specific for NENs as it can be raised in many patients even without NENs. Its specificity for the diagnosis of NENs is low and ranges from 10% to 35%.10 11 The most common reason for spuriously elevated levels is acid suppressive therapy, especially proton pump inhibitor therapy. Hence, it is advised to stop this drug for 1week prior to the test. Histamine receptor antagonists also need to be stopped a day prior to the sample collection and patient needs to be fasting for 12hours.10 11 Other non-endocrine causes for elevated serum chromogranin A levels include impaired renal function, heart failure, pancreatitis, cirrhosis, atrophic gastritis as well as smoking and strenuous exercise prior to testing. It can also be elevated in cancers, such as hepatocellular carcinoma, pancreatic carcinoma, gastric cancer, ovarian and breast cancer among others.10 12 The elevated chromogranin A levels, in our case, were probably because of chronic smoking.
Though octreotide scintigraphy is 70%–95%sensitive for detecting NEN, it is often falsely positive in IPAS as octreotide binds with high affinity to somatostatin receptors on lymphocytes present in splenic tissue.5 Scintigraphy with 99 technetium (Tcm) sulfur colloid or 99Tcm heat-denatured red blood cell (HDRBC) can be used as a confirmatory modality in case of suspicion of accessory spleen. 99Tcm heat-damaged RBC scintigraphy is highly sensitive and specific as splenic tissue traps up to 90% of injected HDRBC and hence, lights up on the scan whereas NEN would not.8
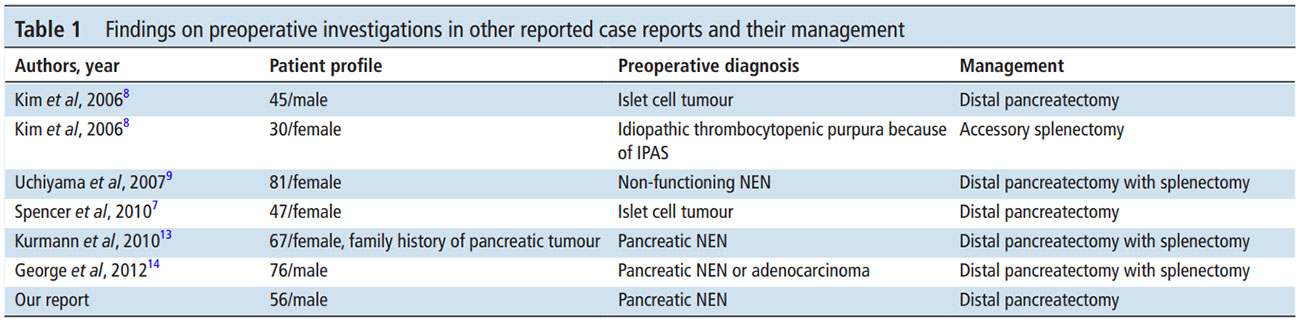
Table 1 Findings on preoperative investigations in other reported case reports and their management
If IPAS is diagnosed on imaging, the patient can be followed up without any intervention. In cases where NEN cannot be ruled out with certainty, endoscopic ultrasound and guided biopsy are the next step to achieve a histological diagnosis. Limited surgical resection as in our case is the final treatment option.2 4 7 Preoperative diagnosis and the management outline of previously reported similar cases is summarised in table 1. 7 8 9 13 14
It can be seen that almost all the patients have been managed for NEN or pancreatic adenocarcinoma. Up to 55% of patients underwent surgical management in a study with 105 patients due to missed diagnosis preoperatively.3 This can possibly change if a suspicion of IPAS arises on CECT/CEUSG/MR characteristics as described above.
Learning points:
- Intrapancreatic accessory spleen is a rare differential diagnosis of hyperenhancing lesion in distal pancreas
- Tumour markers, endoscopic ultrasound-guided biopsy and/ or nuclear medicine imagery armamentarium would help to achieve diagnosis in difficult cases.
- If diagnosed with certainty, unnecessary surgery can be avoided.
- In case of diagnostic uncertainty after all the above tests, surgical resection is the final option.
Contributors:
GSD and NC planned and carried out the review of literature for the case report under conception and design of article by CT and PW. CT provided the pathological inputs. All authors have worked towards the literature review and the management plan of the patient. The report was written by GSD and NC under the critical supervision and inputs of CT and PW
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
References:
- Dodds WJ, Taylor AJ, Erickson SJ, et al. Radiologic imaging of splenic anomalies. AJR Am J Roentgenol 1990;155:805–10.
- Halpert B, Györkey F. Lesions observed in accessory spleens of 311 patients. Am J Clin Pathol 1959;32:165–8.
- Li BQ, Xu XQ, Guo JC. Intrapancreatic accessory spleen: a diagnostic dilemma. HPB 2018;20:1004–11.
- Subramanyam BR, Balthazar EJ, Horii SC. Sonography of the accessory spleen. AJR Am J Roentgenol 1984;143:47–9.
- Brasca LE, Zanello A, De Gaspari A, et al. Intrapancreatic accessory spleen mimicking a neuroendocrine tumor: magnetic resonance findings and possible diagnostic role of different nuclear medicine tests. Eur Radiol 2004;14.
- Ota T, Ono S. Intrapancreatic accessory spleen: diagnosis using contrast enhanced ultrasound. Br J Radiol 2004;77:148–9.
- Spencer LA, Spizarny DL, Williams TR. Imaging features of intrapancreatic accessory spleen. Br J Radiol 2010;83:668–73.
- Kim SH, Lee JM, Han JK, et al. MDCT and superparamagnetic iron oxide (SPIO)- enhanced MR findings of intrapancreatic accessory spleen in seven patients. Eur Radiol 2006;16:1887–97.
- Uchiyama S, Chijiiwa K, Hiyoshi M, et al. Intrapancreatic accessory spleen mimicking endocrine tumor of the pancreas: case report and review of the literature. J Gastrointest Surg 2008;12:1471–3.
- Di Giacinto P, Rota F, Rizza L, et al. Chromogranin A: from laboratory to clinical aspects of patients with neuroendocrine tumors. Int J Endocrinol 2018;2018:1–12.
- Kos-Kudła B, Blicharz-Dorniak J, Strzelczyk J, et al. Diagnostic and therapeutic guidelines for gastro-entero-pancreatic neuroendocrine neoplasms (recommended by the Polish Network of Neuroendocrine Tumours). Endokrynol Pol 2017;68:79–110.
- Tropea F, Baldari S, Restifo G, et al. Evaluation of chromogranin A expression in patients with non-neuroendocrine tumours. Clin Drug Investig 2006;26:715–22.
- Kurmann A, Michel JM, Stauffer E, et al. Intrapancreatic accessory spleen misdiagnosed as a nonsecreting endocrine tumor: case report and review of the literature. Case Rep Gastroenterol 2010;4:210–4.
- George M, Evans T, Lambrianides AL. Accessory spleen in pancreatic tail. J Surg Case Rep 2012;2012:rjs004.