Date: May 8, 2005
Indian J Gastroenterol 2005;24:266-267
Ectopic pancreatic tissue mimicking ampullary tumor
Prasad K Wagle, Guruprasad S Shetty, Mridula Sampat, Kayuri Patel
Department of GI Services, Lilavati Hospital and Research Center, Mumbai; and Department of Pathology, Bhatia Hospital, Mumbai
References:
- Ghazi A, Grossman M. Complications of colonoscopy and polypectomy. Surg Clin North Am 1982;62:889-95.
- Wexner SD, Garbus JE, Singh JJ. A prospective analysis of 13,580 colonoscopies: re-evaluation of credentialing guidelines. Surg Endosc 2001;15:251-61.
- Wherry DC, Zehner H. Colonoscopic fiberoptic endoscopic approach to the colon and polypectomy. Med Ann DC 1974;43:189- 92.
- Ahmed A, Eller PM, Schiffman FJ. Splenic rupture: an unusual complication of colonoscopy. Am J Gastroenterol 1997;92:1201-4.
- Doctor NM, Monteleone F, Zarmakoupis C, Khalife M. Splenic injury as a complication of colonoscopy and polypectomy. Report of a case and review of the literature. Dis Colon Rectum 1987;30:967-8.
- Merchant AA, Cheng EH. Delayed splenic rupture after colonoscopy. Am J Gastroenterol 1990;85:906-7.
Ectopic pancreas is an anomaly in the fusion of the two pancreatic buds where an ectopic rest develops at a place away from the normal site. We report a 70- year-old lady who presented with obstructive jaundice; she was found to have an ampullary tumor highly suggestive of malignancy, for which she underwent pancreatico-duodenectomy. However, histology showed ectopic pancreatic tissue in the ampulla. [Indian J Gastroenterol 2005;24:-266]
Heterotopic pancreas has no anatomic or vascular connection to the main pancreas and results from altered development of the two primitive pancreatic buds that fuse to form the uncinate-head and body-tail of the normal gland. This results in an ectopic rest being dropped from the dorsal pancreatic rudiment, away from the usual location of the body and tail of the pancreas.
A 70-year-old lady presented with upper abdominal pain and mild jaundice for 2 weeks. There was no history of fever or vomiting. There was no previous attack of upper abdominal pain.
She was a known case of atrial fibrillation and hypertension on medications. The liver functions were deranged, with alkaline phosphatase 210 IU/dL and total bilirubin 6.5 mg/dL (direct 4.6). Ultrasonography and CT scan showed dilated common bile duct (CBD) with tapered distal end possibly due to an ampullary tumor, with no liver or distant metastasis. ERCP showed ampullary nodularity with dilated CBD and abrupt distal narrowing. Biliary cytology and biopsy from the ampulla showed highly dysplastic cells suspicious of carcinoma.
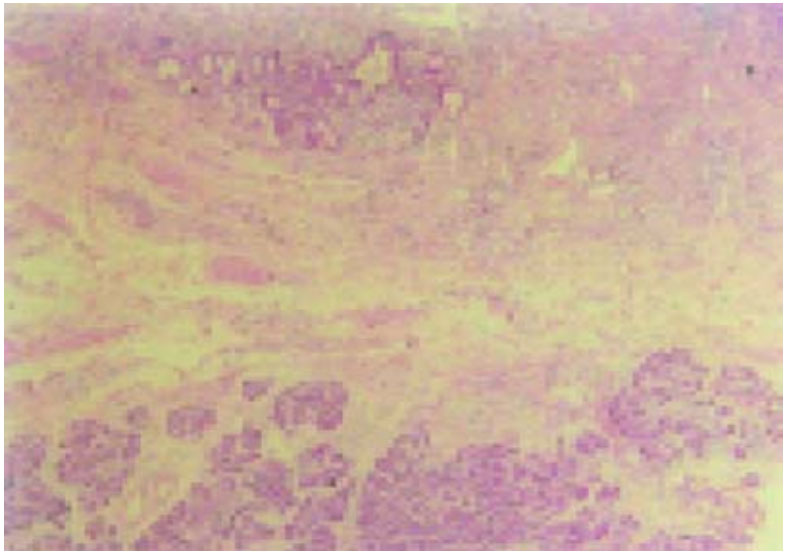
Fig: SPECT co-registered with axial CT showing localized increased uptake in jejunal mesentery.
She was a known case of atrial fibrillation and hypertension on medications. The liver functions were deranged, with alkaline phosphatase 210 IU/dL and total bilirubin 6.5 mg/dL (direct 4.6). Ultrasonography and CT scan showed dilated common bile duct (CBD) with tapered distal end possibly due to an ampullary tumor, with no liver or distant metastasis. ERCP showed ampullary nodularity with dilated CBD and abrupt distal narrowing. Biliary cytology and biopsy from the ampulla showed highly dysplastic cells suspicious of carcinoma.
A decision to perform pancreaticoduodenectomy was taken. Intraoperatively, the liver was normal; there was no ascites or evidence of distant metastasis. Proximal jejunum showed a lesion resembling an extra-pancreatic rest. In the postoperative phase the patient developed atrial fibrillation on the second day, which was treated with medications. She recovered uneventfully thereafter.
Grossly, the ampulla showed evidence of nodularity. The CBD showed a stricture in the distal 1 cm. On microscopy, ampulla showed ulceration in the epithelium. Proliferation of mucous glands with reactive changes was present. Beneath this, ectopic pancreatic tissue was seen within the muscularis propria in the region of the nodularity (Fig). Sections from the CBD showed fibrosis. Tissue from the jejunum showed ectopic pancreatic tissue within the smooth muscle.
The patient is asymptomatic one year later. Ectopic pancreatic tissue (EPT) found during surgical explorations is rare, accounting for < 0.5% of laparotomies, but it is found in 1%- 2% of autopsies.1 EPT is rarely found in children, possibly due to the tendency to remain small along with the slow growth rate. Most symptoms attributable to EPT are not associated with pancreatic disease. Hammarstromm and colleagues2 described 10 cases of ampullary EPT.
Upper gastrointestinal hemorrhage and intestinal obstruction have been ascribed to EPT. Obstruction to the biliary tract has rarely been described.1,3 The presentation of our case was similar to the one reported by Kubota et al. 4
Primary lymph node gastrinoma in jejunal mesentery
Primary gastrinomas have been reported in lymph nodes within the gastrinoma triangle. We report a 56-year-old woman with possible primary lymph node gastrinoma in the jejunal mesentery. Six months after excision of the tumor, she is asymptomatic and serum gastrin level is normal. [Indian J Gastroenterol 2005;24:266-267]
Sporadic gastrinomas are found predominantly to the right of the superior mesenteric artery (SMA) in an area called the gastrinoma triangle. They are frequently extra-pancreatic, multiple, and have a high incidence of lymph node involvement within the triangle. These tumors can be identified and excised for cure in a high percentage of cases.1 Gastrinomas found to the left of the SMA are often within the pancreatic substance, large, infiltrative, and frequently metastatic to the liver at the time of initial diagnosis.2,3
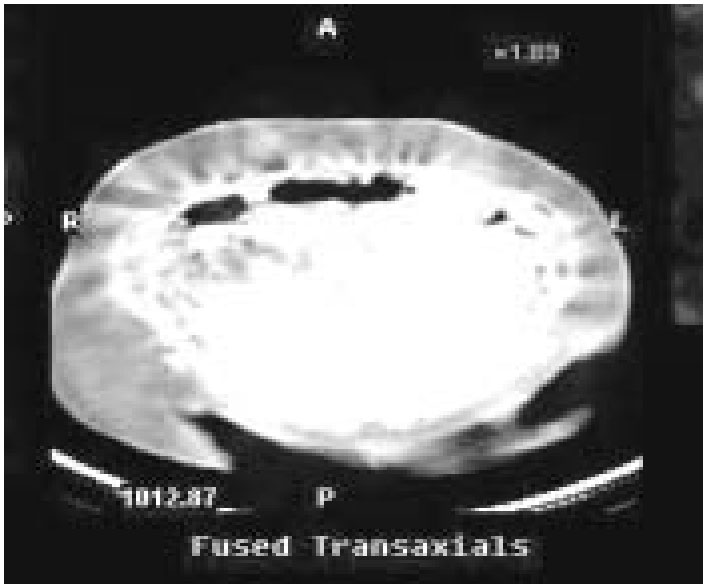
Fig: SPECT co-registered with axial CT showing localized increased uptake in jejunal mesentery
Sporadic gastrinomas are found predominantly to the right of the superior mesenteric artery (SMA) in an area called the gastrinoma triangle. They are frequently extra-pancreatic, multiple, and have a high incidence of lymph node involvement within the triangle. These tumors can be identified and excised for cure in a high percentage of cases.1 Gastrinomas found to the left of the SMA are often within the pancreatic substance, large, infiltrative, and frequently metastatic to the liver at the time of initial diagnosis.2,3
A 56-year-old woman presented with 6-year history of upper abdominal discomfort and loose stools. She had undergone appendicectomy 4 years earlier. Physical examination was unremarkable. She was evaluated for acid-peptic disease and small-bowel diarrhea. At gastroscopy inflammatory changes in the distal esophagus and multiple small ulcers in the second part of the duodenum were seen. Basal serum gastrin level was elevated (960 pg/mL; normal 0-90). She also had elevated parathormone (361 pg/mL; normal 8-74), mildly elevated prolactin (39.3 ng/mL; normal 5-25) and low serum calcium (8.8 mg/ dL; normal >10.4). Plain skull radiograph was unremarkable. CT showed a 2-cm well-enhancing nodule in the jejunal mesentery. 111-indium octreotide scan showed increased uptake in the jejunal mesentery (Fig) and left parathyroid region. Ultrasonography of the thyroid and parathyroid was unremarkable. In view of low serum calcium and negative family history, MEN 1 syndrome was considered unlikely and elevated parathormone was interpreted as a physiological compensatory response.
She was started on calcium supplements. Excision of the gastrinoma was done under general anesthesia. The nodule was located 20 cm from the duodeno-jejunal flexure in the jejunal mesentery free from the bowel wall and mesenteric vessels. Intraoperative ultrasonography of the duodenum and pancreas was normal. Histology revealed a lymph node with its architecture effaced by a tumor. Tumor cells were positive for synaptophysin and chromogranin
Postoperative basal and secretin-stimulated gastrin levels were normal. The patient was symptom-free 6 months later, and basal gastrin levels and serum parathormone were normal.
The gastrinoma triangle is defined as the confluence of the cystic and common bile duct superiorly, second and third portions of the duodenum inferiorly, and the neck and body of the pancreas medially, both dorsally and ventrally. The area of the triangle corresponds to an area of pancreatic development, where the ventral pancreatic bud rotates 180o around the primitive foregut to fuse with the dorsal pancreatic bud.
The existence of primary lymph node gastrinomas is widely debated. There is no embryological basis for the presence of neuroectodermal cells within lymphatic tissue. The current opinion is that the putative stem cell of their genesis is of ventral pancreatic bud origin and that these cells migrate during the posterior rotation of the ventral pancreatic bud to become incorporated in embryonic lymph tissue, later coalescing to form lymph nodes.4 One study reported that chromogranin and synaptophysinpositive cells can be found in abdominal lymph nodes of patients without gastrinomas.5 About 20% of the lymph nodes in the gastrinoma triangle showed synaptophysin-positive cells and 15% had chromogranin in an autopsy series without Zollinger
Ellison syndrome (ZES).4 This histologic study sup-ports the theory that entrapment of neuroendocrine cells during development could lead to development of primary lymph node gastrinomas.
In our patient it is possible that a microscopic primary tumor in an unknown location might have been overlooked; somatostatin receptor scintigra-phy, the most sensitive imaging modality available to localize gastrinomas or their metastases, fre-quently (~50%) misses primary gastrinomas less than 1 cm in diameter and therefore might only localize the accompanying positive lymph nodes.6 We are of the opinion that she had a primary lymph node gastrinoma as six years is too protracted a period for a primary to remain microscopic. Data suggest that patients with tumors to the left of the SMA have shorter duration of symptoms and are often large at presentation.3 Long duration of symp-toms is also in variance with the observed biologic behavior of non ventral pancreatic bud tumors.
The puzzle of the origin of sporadic gastrinomas still seems to be undeciphered. The identification of a possible primary lymph node gastrinoma out-side the gastrinoma triangle is at conflict with cur-rent theories.